
INSTAND, a society for promoting quality assurance in medical laboratories, as an interdisciplinary scientific medical society, has been conducting interlaboratory comparisons worldwide in all areas of in vitro diagnostics since 1970. Learn more about us and our EQAS program in the INSTAND brochure.
Our goals
- Improved diagnostics, therapy monitoring, follow-up, and rehabilitation in medicine for optimal patient care
- Improving the quality of laboratory analyses and their evaluation for the early detection of diseases
- Promotion of research for quality assurance in laboratory medicine
- Advanced training in the quality assurance of medical laboratory diagnostics
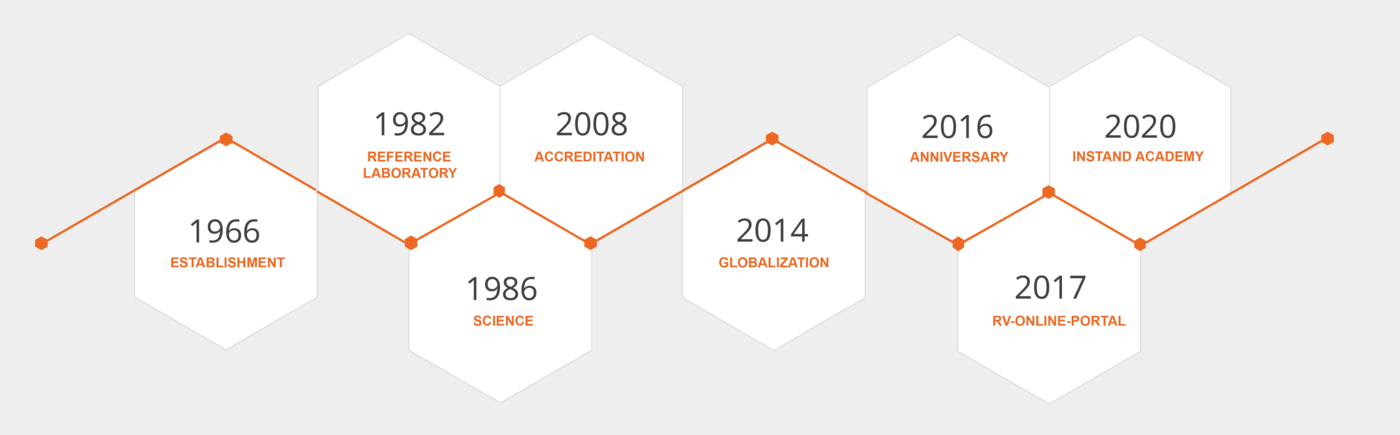
INSTAND Milestones

Foundation of INSTAND
Laboratory analyses in medicine became increasingly important in the post-war period. However, the quality of these analyses had not been tested. The further history is characterised by the continuous development of quality control, also in Germany. From a small association founded in 1966, INSTAND developed into a recognised scientific medical society through many members' voluntary efforts.

Establishment of Own INSTAND Calibration Laboratory
The most crucial objective of the calibration laboratory is to develop reference measurement procedures to evaluate the EQA schemes' results. The standardisation of analytical methods through the establishment of reference measurement procedures is intended to create the necessary comparability among manufacturers and laboratories to protect the patient.
In the INSTAND calibration laboratory, about 28 different reference measurement procedures are continuously monitored and used for the proficiency tests. The spacious calibration laboratories in Düsseldorf are well-staffed and instrumentally well equipped and are therefore a favourable prerequisite for handling research projects.
The reference laboratory was accredited by the German Calibration Service (PTB) according to ISO 15195 and ISO 17025“ in 2008. From 2013 accreditation was carried out by the DAkkS.

First Scientific Publication
As a medical-scientific society, INSTAND aims to contribute to current issues of quality assurance in medical laboratories accessible to an interested professional audience. INSTAND regularly publishes in various media outlets. All publications can be viewed at www.instand-ev.publikationen.
The promotion of research is a primary task of a non-profit scientific-medical society. The promotion of research is realised through several approaches such as project funding and the INSTAND awards (honorary award, research award, dissertation award).

First Accreditation of INSTAND
The accreditation of the reference institution according to the guideline of the German Medical Association and EN 14136:2004 took place in 2008 after a long preparation by the ZLG. The ZLG followed the documents: Directive 98/79/EC, EN 14136:2004, and ISO/IEC Guide 43-1. A surveillance audit by DAkkS took place in 2011.
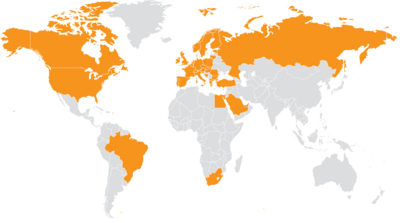
Internationalizaton
Expansion of international activities with the goal of becoming active worldwide
INSTAND offers its proficiency tests worldwide. The INSTAND team is fluent in German, English, Greek, Turkish, and Italian to serve the participants individually.
In various countries, INSTAND cooperates with experienced distributors. The INSTAND partners are selected according to the INSTAND standards and ways to guarantee the INSTAND support in the partner countries.
We continuously focus our activities on expanding our national and international partnership network.

Anniversary
INSTAND celebrates 50th anniversary with
- over 380 EQA schemes
- more than 12,000 participants worldwide
- more than 500 publications in specialized media
- national and international cooperation partners.
RV-Online Portal
INSTAND goes paperless and promotes the trend of sustainability
Since 2017, INSTAND has been offering registration and evaluation for EQA schemes via the RV-ONLINE portal. This platform is continuously adapted and further expanded to ensure a fast and precise evaluation of proficiency tests.

INSTAND Academy
Founding of the INSTAND Academy for continuing education and training of physicians and laboratory personnel
The INSTAND Academy was founded in 2020 as an emerging continuing education platform. Currently, INSTAND offers continuing education, seminars, and microscopy courses in the field of medical laboratories. The aim is to expand the program flexibly and practically to make an essential contribution in the field of quality promotion in medical laboratories.
Our training program is currently only available in German but will be updated with English courses in the near future.
In addition, INSTAND is a member of many national and international professional societies and standardisation committees at DIN, CEN and ISO, and has excellent global scientific networks.
In the Federal Republic of Germany, INSTAND is appointed by the German Medical Association as a reference institution for external quality assurance. INSTAND's versatile EQA program is accredited in all represented disciplines of laboratory diagnostics by the German Accreditation Body (DAkkS) according to DIN EN ISO/IEC 17043:2010.
