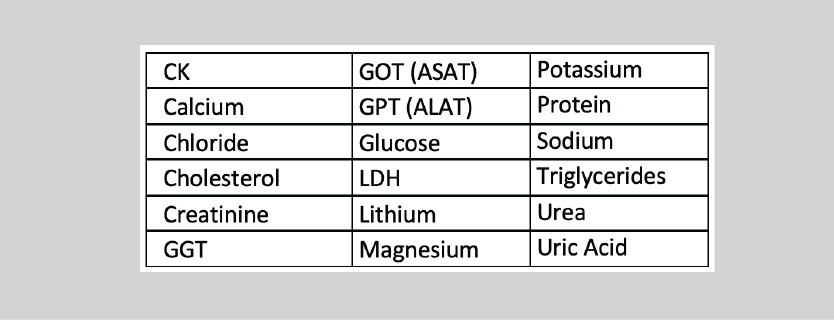
As an additional module in our EQA program, we have introduced a new concept for external quality control in the field of clinical chemistry. Our latest EQA scheme 7100 offers participants the opportunity to carry out external quality control measurements on a monthly basis. This high-frequency program is also characterized by the fact that we provide EQA material in which we have determined target values for 18 measurands using reference measurement procedures in our calibration laboratory accredited by DAkkS in accordance with DIN EN ISO/IEC 17025 and DIN EN ISO 15195. This involves metrological traceability of the target value to a primary standard or a primary reference measurement procedure. These target values can be used to evaluate the monthly measurement results submitted by our participants based on accuracy. The evaluation takes place within two working days.
Measurands:
Timeline:
- Registration for a period of 6 months for monthly participation
- Registration deadline: May 31, 2024
- Sample Shipment: June 18, 2024
- Closing date: from July 2024 on the 7th calendar day of each month
- Evaluation: 2 working days after the closing date (6 monthly certificates and one half-year certificate)
Sample Material:
- 12 samples á 5ml per registration (6 sample sets with 2 samples each) for monthly participation
- lyophilised serum